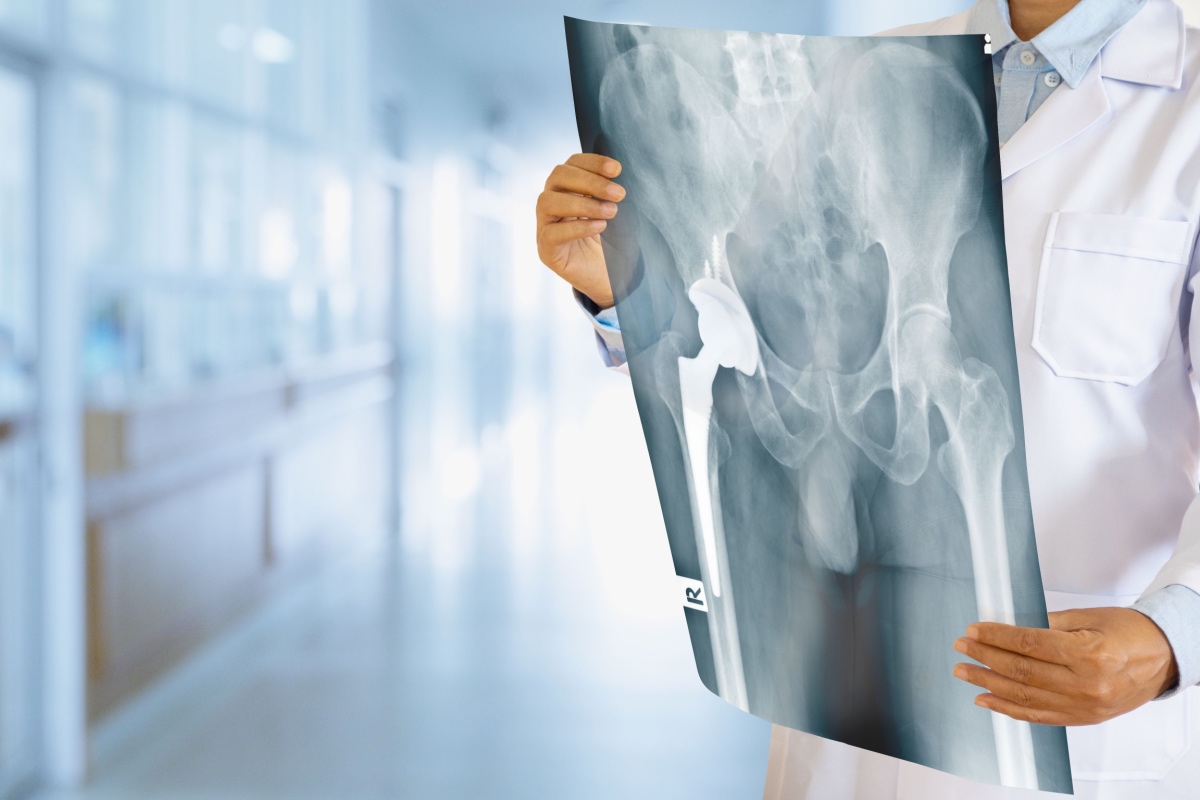
Wright’s Profemur hip replacement system was created to provide patients with a custom-fitted hip joint, allowing them better mobility after surgery.
The device has six interchangeable neckpieces in multiple lengths that can be fitted onto the stem to fit patients of all shapes and sizes, especially those undergoing complex revision surgeries.
Unfortunately, this modular design has a metal stem and metal neck components, causing complications that have been proven to increase the risk of early failure.
Wright Medical marketed the Profemur-Z hip as a more durable product than other artificial hip implants and one uniquely suited for persons with active lifestyles.
The Profemur-Z system consists of three separate components that are assembled during surgery: a femoral head, a modular neck, and a femoral stem. Before 2009, the Profemur-Z hip system’s design included a titanium modular neck adapter and stem.
Over 10,000 patients across America received the Profemur Z with a titanium neck in hip replacement surgery.
In 2009, the Australian Orthopaedic Association found that one component of the hip system, the Profemur-Z femoral stem, failed at an unacceptable rate, requiring revision surgery in one out of every nine patients.
As with many other metal-on-metal modular hip implants, such as the Stryker ABG II, the Wright Medical Profemur system was found to wear at the joint, causing potentially toxic metal shards to be released into the patient’s system.
When these metals are released into the bloodstream, they can cause a severe medical condition called metallosis. Metallosis can affect the heart, nerves, kidneys, and thyroid. Symptoms may include:

Years after multiple lawsuits were filed due to injuries caused by unexpected hip replacement fractures that led to broken bones and more severe injuries, the Food and Drug Administration in 2015 filed a recall of a Profemur hip replacement system.
The devices were prone to sudden fractures at the module neck, which resulted in a Class 1 recall. A Class 1 recall is the most severe type of FDA-issued recall associated with the potential risk of severe injury or death.
The Profemur stems, manufactured by Wright Medical Group, Inc., were used as part of modular neck hip implants to replace damaged portions of the hip joint.
The recalled components were prone to various faults, including corrosion, fretting, and the accumulation of metal debris in the body. The problems occurred when the hip replacements broke, causing broken bones and the need for revision surgeries.
Potential problems included neurovascular damage, hematoma, hemorrhage, or death, according to the FDA.
The recall affected more than 10,000 devices manufactured and distributed between 2009 and 2015.
The first lawsuit was tried in Los Angeles Superior Court in 2015, and it resulted in a more than $1 million recovery. Since then, there have been over 1,200 lawsuits involving Profemur defects.
In 2017, the Tennessee-based device maker offered to settle hundreds of personal injury lawsuits from people across the country who alleged that their Wright implants caused medical complications.
Many of them required revision surgeries to remove the device and treat their injuries.
There is a great deal of litigation against Wright Medical Technology and many other hip implant device manufacturers. If you or a loved one is experiencing health problems related to your hip implant, you have options.
It is important for you to understand all of these options, know what steps to take to protect your health, and ensure you have the tools you need to protect your rights. Contact us today!

Attorney Timothy Manchin established the Manchin Injury Law Group in 2011 after his law partner of more than 25 years became a West Virginia circuit court judge. His focus is on helping individual clients and entire families victimized by negligent acts.
We offer a free initial consultation at our office in the Manchin Professional Building — our home since 1983 — conveniently located in Fairmont.
If you are unable to visit our firm, we can come to your home or hospital room.
Fill out the form below to get in touch!